Modified True/False
Indicate
whether the statement is true or false. If false, change the identified word or phrase to make
the statement true.
|
|
|
1.
|
The electrons that are involved in the chemical bonding are called
oxidation electrons. _________________________
|
|
|
2.
|
The type of chemical bond formed by the sharing of electrons is the ionic
bond. _________________________
|
|
|
3.
|
The most stable number of valence electrons an element in the third period may
possess is 18 electrons. _________________________
|
|
|
4.
|
Covalent bonds are more likely to form between two atoms when the separation
between them on the periodic table is small. _________________________
|
|
|
5.
|
Strong electron donors are found on the left side of the periodic table.
_________________________
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
6.
|
Atoms of elements in period 2 and period 3 are similar because the largest
number of valence electrons their atoms may contain in the outermost energy level is:
|
|
|
7.
|
Bonding between atoms produces an outermost energy level for each atom filled
with:
a. | 1 electrons | b. | 8 electrons | c. | 16
electrons | d. | 32 electron |
|
|
|
8.
|
The diagram below represents a chlorine atom. The small dark circles represent
electrons surrounding the nucleus. 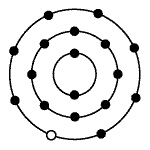 Which of the atoms
pictured would be most likely to bond with a single chlorine atom? 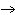
|
|
|
9.
|
In the atom represented below, the small dark circles represent
electrons. 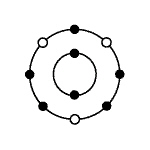 The number of valence electrons in the
atom pictured is:
|
|
|
10.
|
The largest number of valence electrons an atom in the first period can have
is:
|
|
|
11.
|
A molecule of water, formed when two hydrogen atoms (oxidation number 1+) and
one oxygen atom (oxidation number 2-) bond, has an oxidation number of:
|
|
|
12.
|
The oxidation number for halogens is:
|
|
|
13.
|
 The diagram which best represents the
Lewis dot diagram of a chlorine atom is:
|
|
|
14.
|
The atom of the element most likely to bond with a single atom of fluorine,
atomic # 9 is:
a. | lithium, atomic #3 | b. | beryllium, atomic #4 | c. | boron, atomic
#5 | d. | carbon, atomic #6 |
|
|
|
15.
|
The number of valence electrons in an atom of aluminum, atomic #13 is:
|
|
|
16.
|
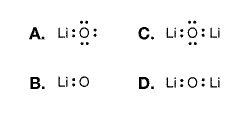 The best Lewis dot diagram representation
of the bonding between lithium and oxygen atoms is:
|
|
|
17.
|
The most common oxidation number for an element with 16 electrons is:
|
|
|
18.
|
An ionic bond is most likely to form between:
a. | carbon and oxygen atoms. | b. | lithium and fluorine atoms. | c. | two oxygen
atoms. | d. | nitrogen and oxygen atoms. |
|
|
|
19.
|
Covalent bonds would most likely form between:
a. | an alkali metal and helium. | b. | magnesium and a halogen. | c. | a metalloid and
oxygen. | d. | sodium and chlorine. |
|
Completion
Complete each
statement.
|
|
|
Select the correct term to complete each sentence. There are extra terms in
the list.
| bond | oxidation | mixture | | atom | covalent bond | molecule | | formula | ion | element | | | |
|
|
|
20.
|
The number indicating the charge on an atom when
an electron is lost, gained or, shared is called the ____________________.
|
Short Answer
|
|
|
21.
|
What is the oxidation number of manganese (Mn) in the compound, MnO2,
if oxygen (O) has an oxidation number of -2?
|
|
|
22.
|
Barium has an oxidation number of +4 and oxygen has an oxidation of -2.
a.
What is the chemical formula for barium oxide?
b. What is the total charge for barium
oxide?
|
|
|
23.
|
What is the formula for mercury bromide? Mercury (Hg) has an oxidation number of
+2 and bromine (Br) has an oxidation number of -1.
|
Problem
|
|
|
24.
|
An oxygen atom is represented below. The small dark circles represent electrons
surrounding the nucleus. 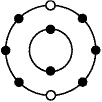 What is the number of valence electrons in
the oxygen atom?
|
|
|
25.
|
Draw a Lewis dot diagram to represent an atom of silicon.
|
|
|
26.
|
If an iron atom loses 2 electrons, what is the oxidation number for the ion that
is formed?
|
|
|
27.
|
Draw a Lewis dot diagram to represent the compound formed when calcium atoms
bond with fluoride atoms to form calcium fluoride.
|
Essay
|
|
|
28.
|
The neon atom pictured below does not bond with other atoms frequently.
Why? 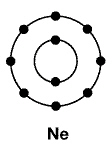
|
|
|
29.
|
What determines whether atoms will form covalent or ionic bonds?
|