Modified True/False
Indicate
whether the statement is true or false. If false, change the identified word or phrase to make
the statement true.
|
|
|
1.
|
Properties such as boiling point, phase, density, and specific heat are known as
chemical properties. _________________________
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
2.
|
As the atomic numbers of elements increase, their atomic weights:
a. | increase. | b. | decrease. | c. | remain the
same. | d. | are unrelated to a change in their atomic numbers. |
|
|
|
3.
|
Steel can be described as all of the following EXCEPT a(n):
a. | mixture. | b. | conductor. | c. | alloy. | d. | element. |
|
|
|
4.
|
Of the following elements, the one most essential to life on Earth is:
a. | oxygen. | b. | iron. | c. | carbon. | d. | silicon. |
|
|
|
5.
|
Elements used to make semiconductors for computers include germanium and:
a. | oxygen. | b. | iron. | c. | carbon. | d. | silicon. |
|
|
|
6.
|
The most abundant element in Earth’s crust is:
a. | oxygen. | b. | silicon. | c. | carbon. | d. | nitrogen. |
|
|
|
7.
|
An element essential to the manufacture of DNA and glow-in-the-dark plastic
is:
a. | fluorine. | b. | phosphorus. | c. | sulfur. | d. | nitrogen. |
|
|
|
8.
|
Of the following, the best insulator is the element:
a. | zinc. | b. | chromium. | c. | sulfur. | d. | magnesium. |
|
|
|
9.
|
The element that is shiny in appearance, quite dense and liquid at room
temperature is:
a. | silver. | b. | aluminum. | c. | mercury. | d. | zinc. |
|
|
|
10.
|
When the outermost energy level of an atom is filled, at room temperature, the
resulting element:
a. | is often quite dense. | b. | does not interact strongly with other
atoms. | c. | occurs as a gas. | d. | generally occurs as a
solid. |
|
|
|
11.
|
Most elements with high melting points have:
a. | filled energy levels. | b. | half-filled energy levels. | c. | empty energy
levels. | d. | no other common characteristics. |
|
|
|
12.
|
A characteristic of elements that demonstrate the strongest periodicity
is:
a. | boiling point. | b. | thermal conductivity. | c. | atomic
weight. | d. | electrical insulation. |
|
Completion
Complete each
statement.
|
|
|
Select the correct term to complete each sentence. There are extra terms in
the list.| groups | rows | periods | | noble | halogens | metalloids | | alkali | nonmetals | periodicity | | | |
|
|
|
13.
|
When reading across rows of the period table, patterns of chemical and physical
properties tend to be repeated. This pattern is called ____________________
|
Short Answer
|
|
|
14.
|
Name three elements that are good conductors of electricity.
|
Problem
|
|
|
15.
|
The diagram below represents the periodic table of elements. It is shaded in
three tones to represent areas in the periodic table where non-metals, metals and metalloids are
located. 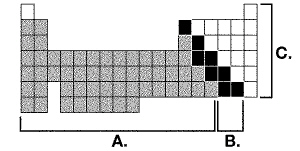 Identify the areas in which the three
types of elements would be found on the periodic table by placing the letters A, B and C next to the
element types. | | Non-metal _____ | Metal _____ | Metalloid _____ | | | | |
|
Essay
|
|
|
16.
|
Give two reasons silicon is of economic importance.
|
|
|
17.
|
What are alloys and why are they widely used? Include two examples of alloys in
your answer.
|
|
|
18.
|
Most elements are found as solids. One group, the noble gases, is exceptional
because the entire group occurs as gases under normal conditions. Explain why this is so.
|