Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
The diagram below represents a spectrum. Each line is a different color of
light. The bright colored lines represent: 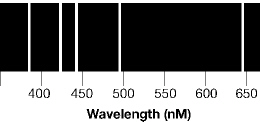 a. | the diameter of several isotopes. | b. | light of different energies from an
element. | c. | atoms that are radioactive. | d. | values for the strong force for several
isotopes. |
|
|
|
2.
|
Which statement below does NOT correctly describe the relationship
between electrons and their energy levels?
a. | An electron’s energy must match one of the energy levels in an
atom. | b. | Each energy level can hold only a certain number of electrons. | c. | Electrons added to
an atom settle into unfilled positions nearest the nucleus. | d. | The electrons with
the greatest energy are located closest to the nucleus. |
|
|
|
3.
|
The diagram below represents the electron energy levels for the first two levels
of an atom. 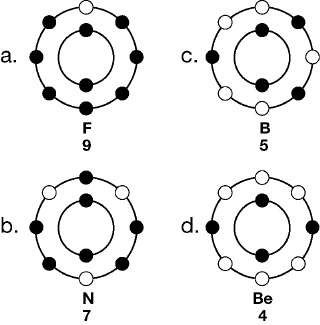 The diagram that would best represent an
atom whose nucleus contains 5 protons and 4 neutrons is:
|
|
|
4.
|
Electrons can have only certain amounts of energy. Which phrase includes ideas
that best support this statement?
a. | Radioactive elements are unstable. | b. | All atoms of the same element contain the same
number of protons. | c. | Only specific colors of light can be given off
by each element. | d. | The mass number of each element is the sum of protons and
neutrons. |
|
|
|
5.
|
An atom contains 11 electrons. If a 12th electron were added to the atom, the
energy level into which it would be placed is energy level:
|
Completion
Complete each
statement.
|
|
|
Select the correct term to complete each sentence. There are extra terms in
the list.| alpha | beta | spectroscope | | neutral | charged | isotopes | | 2 | 6 | 8 | | strong
nuclear | gravitational | weak | | | |
|
|
|
6.
|
The instrument used to separate the light given off by electrons into different
colors is called a _________________________.
|
|
|
7.
|
The number of electrons that may be held in the third energy level of an atom is
____________________.
|
Short Answer
|
|
|
8.
|
Of the three sub-atomic particles: electrons, protons and neutrons, which
determines most of the properties of an element?
|
|
|
9.
|
Two electrons, A and B, are temporarily raised to different energy levels. As
they fall back toward the nucleus, A emits green light and B emits red light. Which electron has more
energy before falling toward the nucleus?
|
Problem
|
|
|
10.
|
Using the diagram below as a model, represent the electron energy levels of a
fluorine atom, atomic number 9. 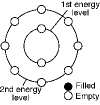
|
Essay
|
|
|
11.
|
When a gas is heated, it may give off light. Explain how a scientist might
identify the element or elements from which the gas is made.
|
|
|
12.
|
What causes an electron to emit light?
|