Modified True/False
Indicate
whether the statement is true or false. If false, change the identified word or phrase to make
the statement true.
|
|
|
1.
|
The reaction that breaks down water into hydrogen and oxygen using electricity
is a single-displacement reaction. _________________________
|
|
|
2.
|
A series of addition reactions that joins small molecules into large chain
molecules is known as polymerization. _________________________
|
|
|
3.
|
A solid product that comes out of solution in a chemical reaction is called a
pollutant. _________________________
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
4.
|
Polymers are large molecules made up of repeating segments. They are formed
through a series of:
a. | addition reactions. | b. | single-displacement
reactions. | c. | combustion reactions. | d. | decomposition
reactions. |
|
|
|
5.
|
The force that holds neutrons and protons together in the nucleus is known
as:
a. | the electronegative force. | b. | hydrogen bonding. | c. | the strong nuclear
force. | d. | the weak nuclear force. |
|
|
|
6.
|
When you activate an instant cold pack, water mixes with a chemical and the pack
gets very cold. This is an example of a(n):
a. | endothermic reaction. | b. | exothermic reaction. | c. | combustion
reaction. | d. | a physical change. |
|
|
|
7.
|
What would be the most likely product(s) of the following reaction? (Assume that
the activation energy has been supplied). H2 + O2 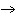 ____ ____a. | H3 + O3 | b. | H2O | c. | O3H3 | d. | No reaction would
occur. |
|
|
|
8.
|
A test tube full of alcohol is open to the air. Alcohol burns very well, but the
alcohol in the test tube does not start burning by itself because:
a. | there is no oxygen in the test tube. | b. | the combustion reaction requires an activation
energy to get started. | c. | the alcohol is a liquid and only burns when it
is a gas. | d. | carbon monoxide in the air prevents combustion from
starting. |
|
|
|
9.
|
Classify the following reaction: 3CuSO4 + 2Al 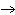 Al2(SO4)3 + 3Cu
Al2(SO4)3 + 3Cua. | Addition | b. | Single-displacement | c. | Combustion | d. | Decomposition |
|
|
|
10.
|
Classify the following reaction: 2C8H18 +
25O2 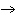 16CO2 + 18H2O 16CO2 + 18H2Oa. | Addition | b. | Single-displacement | c. | Combustion | d. | Decomposition |
|
|
|
11.
|
Classify the following reaction: Cu(OH)2 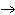 CuO
+ H2O CuO
+ H2Oa. | Addition | b. | Single-displacement | c. | Combustion | d. | Decomposition |
|
|
|
12.
|
Which of the following statements about nuclear reactions is FALSE?
a. | Nuclear reactions involve only the outer electrons of an atom. | b. | Nuclear reactions
may change atoms of one element into atoms of a different element | c. | Nuclear reactions
may change an isotope into a different isotope of the same element. | d. | Nuclear reactions
involve more energy than chemical reactions. |
|
|
|
13.
|
Nuclear reactions can convert mass into:
a. | friction. | b. | weight. | c. | energy. | d. | gravity. |
|
|
|
14.
|
Which of the following statements about chemical reactions is FALSE?
a. | Chemical reactions rearrange atoms into different combinations of
molecules. | b. | Chemical reactions involve the outer electrons of the atoms. | c. | Chemical reactions
involve a smaller amount of energy than nuclear reactions. | d. | Chemical reactions
can convert mass into energy. |
|
|
|
15.
|
What is the by-product of the combustion reaction given
below: C6H12O6 + 6O2 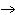 6CO2
+ 6H2O 6CO2
+ 6H2Oa. | Energy is absorbed. | b. | Energy is released. | c. | C6H12O6 | d. | Sugar |
|
Completion
Complete each
statement.
|
|
|
Select the correct term to complete each sentence. There are extra terms in
the list.| 14 | 16 | 7 | | exothermic | balanced | endothermic | | activation energy | reaction | chemical | | | |
|
|
|
16.
|
In a chemical reaction, the energy needed to break chemical bonds in the
reactants is called the ______________________________ (two words).
|
|
|
17.
|
A chemical reaction that releases more energy than it uses is
____________________.
|
Short Answer
|
|
|
Answer the following questions based on the balanced combustion reaction given
below. C6H12O6 + 6O2 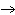 6CO2 + 6H2O
6CO2 + 6H2O + energy
|
|
|
18.
|
Based on the information given in the chemical equation, what temperature change
would you expect in the reaction? Why would you expect this?
|
|
|
19.
|
Is this an endothermic or exothermic reaction?
|
|
|
20.
|
Combustion reactions are a part of our everyday lives. List two or more that you
know about.
|
|
|
Answer the following questions based on dissolving ammonium nitrate in water.
The balanced equation is given below.
NH4NO3(s) + H2O(l) +
energy ® NH4+(aq) +
NO3-(aq) + H2O(l)
|
|
|
21.
|
Based on the information given in the chemical equation, what temperature change
would you expect in the reaction? Why would you expect this?
|
|
|
22.
|
Is this an endothermic or exothermic reaction?
|
Essay
|
|
|
23.
|
How are chemical reactions and nuclear reactions different? Describe at least
three differences.
|
|
|
24.
|
Gas pumps often display a warning sign that says “Do not get into your car
while you are filling the tank. You may create a static electric spark that could cause gasoline
vapors to ignite, resulting in an explosion.”
Explain why the gasoline vapors
don’t start burning as soon as they come in contact with oxygen in the air. What would the
static electric spark provide?
|