Modified True/False
Indicate
whether the statement is true or false. If false, change the identified word or phrase to make
the statement true.
|
|
|
1.
|
The products are the substances formed as the result of a chemical
reaction. _________________________
|
|
|
2.
|
Melting ice is an example of a chemical change.
______________________________
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
3.
|
When a chemical change occurs:
a. | atoms are rearranged. | b. | the law of conservation of mass is always
obeyed. | c. | the chemical properties of new substances are different from the ones you started
with. | d. | All of the above |
|
|
|
4.
|
Your teacher mixes a liquid and a solid in a beaker. Which of the following is
NOT evidence that a chemical change has occurred?
a. | A drop of liquid that spills on the lab table quickly evaporates. | b. | The temperature of
the mixture changes from 20°C to 10°C. | c. | Bubbles form in the beaker. | d. | The mixture changes
color. |
|
|
|
5.
|
The principle of conservation of mass is satisfied in a chemical equation
when:
a. | the same kinds of atoms appear in the reactants and products. | b. | the same number of
each type of atom appears on the equation’s reaction side and product side. | c. | the sum of the
coefficients on the reactant side is the same as the sum of the coefficients on the product
side. | d. | each atom on the product side has the same subscript that it had on the reactant
side. |
|
|
|
Answer the following questions about the chemical reaction for the formation of
rust: Fe + O2 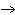 Fe2O3 Fe2O3
|
|
|
6.
|
Identify the reactant(s):
a. | Fe and Fe2O3 | b. | Fe and O2 | c. | Fe2O3 | d. | O2 and
Fe2O3 |
|
|
|
7.
|
Identify the product(s):
a. | Fe and Fe2O3 | b. | Fe and O2 | c. | Fe2O3 | d. | O2 and
Fe2O3 |
|
|
|
8.
|
Is the chemical reaction for the formation of rust balanced? If not, select the
correct equation from the ones listed below.
a. | Yes, it is balanced. | b. | No, this is balanced: 2Fe + O2 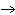 Fe2O3 Fe2O3 | c. | No, this is balanced: 4Fe + 3O2 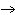 2Fe2O3 2Fe2O3 | d. | No, this is balanced: 3Fe + 2O2 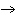 3Fe2O3 3Fe2O3 |
|
|
|
Answer the following questions about the chemical reaction for the combustion of
methane gas: CH4 + O2 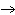 CO2 +
H2O CO2 +
H2O
|
|
|
9.
|
Identify the reactants.
a. | CO2 and H2O | b. | CH4 and
O2 | c. | O2 and H2O | d. | CH4 and
CO2 |
|
|
|
10.
|
Identify the products.
a. | CO2 and H2O | b. | CH4 and
O2 | c. | O2 and H2O | d. | CH4 and
CO2 |
|
|
|
11.
|
Is the chemical reaction for the combustion of methane balanced? If not, select
the correct equation from the ones listed below.
a. | Yes, it is balanced. | b. | No, this is balanced: CH4 +
O2 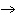 CO2 +
4H2O CO2 +
4H2O | c. | No, this is balanced: CH4 + 2O2 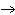 CO2
+ 2H2O CO2
+ 2H2O | d. | No, this is balanced: CH4 +
4O2 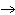 CO2 +
H2O CO2 +
H2O |
|
|
|
12.
|
Balance the following equation to demonstrate the conservation of atoms in a
reaction. Choose the answer that provides the correct coefficients for each reactant and
product: ____ Al2O3 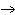 ____ Al+ ____
O2 ____ Al+ ____
O2a. | (2, 4, 3) | b. | (4, 3, 2) | c. | (3, 2,
4) | d. | (2, 3, 4) |
|
|
|
13.
|
A reaction is exothermic if forming the new bonds:
a. | requires more energy than it took to break the old bonds. | b. | takes the same
amount of energy as was needed to break the old bonds. | c. | absorbs energy from the system, causing a
temperature drop. | d. | releases more energy than it took to break the
old bonds. |
|
|
|
14.
|
In which of the following situations does water undergo a change in physical
properties?
a. | The bathroom fills with steam when you take a hot shower. | b. | You pour a glass of
water from the faucet. | c. | Water is broken down to yield H2 and
O2. | d. | When hydrogen is used as a fuel for rockets, water is a
product. |
|
|
|
15.
|
Balance the following equation to demonstrate the conservation of atoms in a
reaction. Choose the answer which provides the correct coefficients for each reactant and
product. ____ CS2 + ____ O2 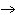 ____ CO2 + ____
SO2 ____ CO2 + ____
SO2a. | (1, 3, 1, 2) | b. | (1, 2, 1, 1) | c. | (1, 6, 1,
2) | d. | (2, 6, 2, 2) |
|
|
|
16.
|
If you designed your own experiment to prove the law of conservation of mass,
what conditions would be required?
a. | You must find the mass of the reactants before the reaction and find the mass of the
products after the reaction. | b. | You must find the mass of the reactants before
the reaction, find the mass of the products after the reaction, AND perform the reaction in a closed
system. | c. | You must choose a combustion reaction. | d. | You must use baking soda and vinegar because
the law applies only to these substances. |
|
Completion
Complete each
statement.
|
|
|
Select the correct term to complete each sentence. There are extra terms in
the list.| 14 | 16 | 7 | | exothermic | balanced | endothermic | | activation energy | reaction | chemical | | | |
|
|
|
17.
|
A(n) ____________________ equation for a chemical reaction correctly preserves
the number and type of atoms on both sides of the reaction.
|
|
|
18.
|
The chemical formula 2C3H7OH means you have 6 atoms of
carbon, ____________________ atoms of hydrogen, and 2 atoms of oxygen.
|
Short Answer
|
|
|
19.
|
For the following antacid reaction, list the reactants and the
products:
2HCl + CaCO3 ® CaCl2 +
CO2 + H2O
|
|
|
20.
|
Your teacher mixes two clear liquids in a beaker. Name three changes in the
mixture that would provide evidence that a chemical reaction had occurred.
|
Problem
|
|
|
21.
|
Balance the following chemical equation:
Al + O2 ® Al2O3
|
|
|
22.
|
Balance the following chemical equation:
HgO ® Hg + O2
|
|
|
23.
|
Balance the following equation:
K + H2O ® KOH + H2
|
|
|
24.
|
Write the balanced equation for the following reaction:
The fuel used in gas
grills is called propane (C3H8). Propane reacts with oxygen gas (O2)
found in the air to produce carbon dioxide gas (CO2) and water vapor
(H2O).
|
Essay
|
|
|
25.
|
When you mix baking soda and vinegar, the mixture bubbles violently and the
temperature drops. Is this a chemical change or a physical change? Provide evidence to support your
answer.
|
|
|
26.
|
Explain the statement, “Chemical reactions conserve mass.”
|
|
|
27.
|
When balancing chemical equations, why can’t you change the subscripts of
the molecules?
|